-
-
Sputtering Targets
- Element Sputtering Targets
- Alloy Sputtering Targets
- Oxide Sputtering Targets
- Nitride Sputtering Targets
- Carbide Sputtering Targets
- Fluoride Sputtering Targets
- Silicide Sputtering Targets
- Sulfide Sputtering Targets
- Selenide Sputtering Targets
- Telluride Sputtering Targets
- Antimonide Sputtering Targets
- Phosphide Sputtering Targets
- Special Sputtering Targets Customization
- Sputtering Targets List
-
Evaporation Materials
- Element Evaporation Materials
- Alloy Evaporation Materials
- Oxide Evaporation Materials
- Nitride Evaporation Materials
- Carbide Evaporation Materials
- Fluoride Evaporation Materials
- Phosphide Evaporation Materials
- Telluride Evaporation Materials
- Selenide Evaporation Materials
- Sulfide Evaporation Materials
- Other Evaporation Materials
- Evaporation Materials List
- Compound Semiconductor Materials
- Rare Earth Materials
- Special Alloy
-
Crucible customization
-
Sputtering Targets
- H氢
- He氦
- Li锂
- Be铍
- B硼
- C碳
- N氮
- O氧
- F氟
- Ne氖
- Na钠
- Mg镁
- Al铝
- Si硅
- P磷
- S硫
- Cl氯
- Ar氩
- K钾
- Ca钙
- Sc钪
- Ti钛
- V钒
- Cr铬
- Mn锰
- Fe铁
- Co钴
- Ni镍
- Cu铜
- Zn锌
- Ga镓
- Ge锗
- As砷
- Se硒
- Br溴
- Kr氪
- Rb铷
- Sr锶
- Y钇
- Zr锆
- Nb铌
- Mo钼
- Tc碍
- Ru钌
- Rh铑
- Pd钯
- Ag银
- Cd镉
- In铟
- Sn锡
- Sb锑
- Te碲
- I碘
- Xe氙
- Cs铯
- Ba钡
- LaLu镧系
- Hf铪
- Ta钽
- W钨
- Re铼
- Os锇
- Ir铱
- Pt铂
- Au金
- Hg汞
- Tl铊
- Pb铅
- Bi铋
- Po钋
- At砹
- Rn氡
- La镧
- Ce铈
- Pr镨
- Nd钕
- Pm钷
- Sm钐
- Eu铕
- Gd钆
- Tb铽
- Dy镝
- Ho钬
- Er铒
- Tm铥
- Yb镱
- Lu镥
点击可查看包含对应元素的相关产品信息
科泰新材料可提供“元索周期表”近乎全元素(除放射性元素外)的任意组合材料定制,
部分产品未及时更新,特殊定制需求请咨询客服。
- Al铝
- Ar氩
- As砷
- Ag银
- Au金
- At砹
- Be铍
- B硼
- Br溴
- Ba钡
- Bi铋
- C碳
- Cl氯
- Ca钙
- Cr铬
- Co钴
- Cu铜
- Cd镉
- Cs铯
- Ce铈
- Dy镝
- Eu铕
- Er铒
- F氟
- Fe铁
- Ga镓
- Ge锗
- Gd钆
- H氢
- He氦
- Hf铪
- Hg汞
- Ho钬
- In铟
- I碘
- Ir铱
- K钾
- Kr氪
- Li锂
- LaLu镧系
- La镧
- Lu镥
- Mg镁
- Mn锰
- Mo钼
- N氮
- Ne氖
- Na钠
- Ni镍
- Nb铌
- Nd钕
- O氧
- Os锇
- P磷
- Pd钯
- Pt铂
- Pb铅
- Po钋
- Pr镨
- Pm钷
- Rb铷
- Re铼
- Rn氡
- Ru钌
- Rh铑
- Si硅
- S硫
- Sc钪
- Se硒
- Sr锶
- Sn锡
- Sb锑
- Te碲
- Sm钐
- Ti钛
- Tc碍
- Ta钽
- Tl铊
- Tb铽
- Tm铥
- V钒
- W钨
- Xe氙
- Yb镱
- Y钇
- Zn锌
- Zr锆
当前位置:首页 >> Products >> Sputtering Targets >> Element Sputtering Targets
- 产品类别
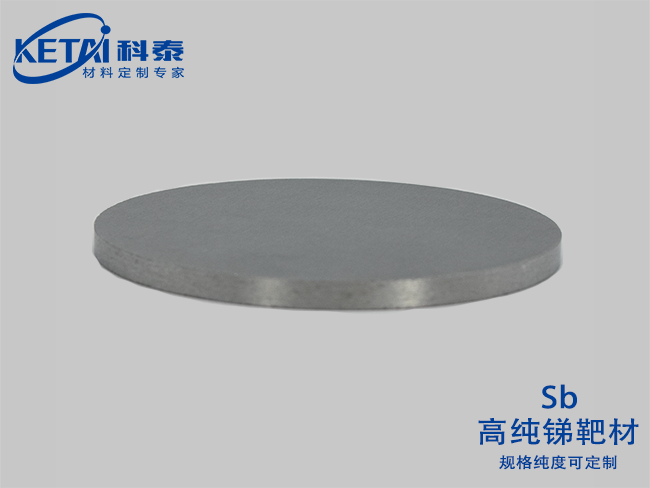
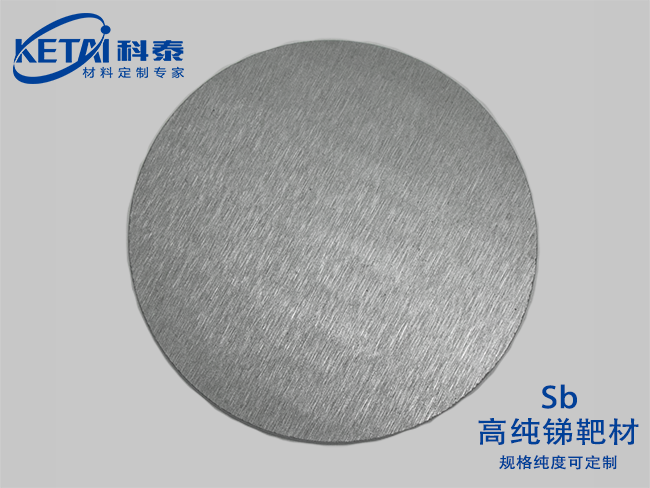
Antimony sputtering targets(Sb)
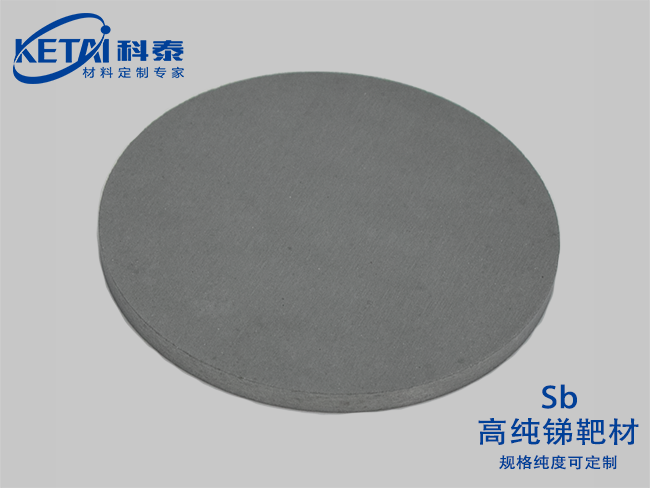
| Antimony sputtering targets(Sb)基本信息 | |
| 分子式 | Sb |
| 纯度 | 99.999% |
| CAS号 | 7440-36-0 |
| 摩尔质量 | 121.760 |
| 密度 | 6.697 g/cm³ |
| 熔点 | 630 ℃ |
| 沸点 | 1635 ℃ |
| 溶解性(水) | |
Antimony sputtering targets(Sb)产品概况
Antimony is a gray metal with a silver luster and a Mohs hardness of 3. Many objects have the characteristics of thermal expansion and contraction. For example, our commonly used thermometers utilize the principle of mercury thermal expansion and contraction. However, antimony is different in that it shrinks and expands in a certain temperature range.
Antimony is a nitrogen group element (group 15) with an electronegativity of 2.05 (Pauline scale). According to the periodic law of elements, its electronegativity is greater than that of tin and bismuth, but smaller than that of tellurium and arsenic. Antimony is stable in air at room temperature, but can react with oxygen to form antimony trioxide when heated. Antimony does not react with acids under normal conditions.
There are known four allotropes of antimony - a stable metal antimony and three metastable antimony (explosive antimony, black antimony, and yellow antimony). Metallic antimony is a brittle silver white shiny metal. "When the molten antimony is slowly cooled, the metal antimony will form a triangular crystal system, which is heteromorphic to the gray allotrope of arsenic.". The rare explosive form of antimony can be produced by electrolysis of antimony trichloride. Scratching it with a sharp tool will cause an exothermic chemical reaction, emitting white smoke and forming metallic antimony. If you grind it with a pestle in a mortar, a violent explosion will occur. Black antimony is formed by the rapid cooling of metal antimony vapor. Its crystal structure is the same as that of red phosphorus and black arsenic, and it is prone to oxidation and even spontaneous combustion in oxygen. When the temperature drops to 100 ℃, it gradually transforms into a stable crystal form. Yellow antimony is the most unstable type and can only be obtained by oxidation of hydrogen antimonide at - 90 ℃. Under the action of this temperature and ambient light, metastable allotropes can be converted into more stable black antimony.
The structure of metallic antimony is a layered structure (spatial group: R3m), and each layer contains a connected folded six-membered ring structure. The nearest and next nearest antimony atoms form a deformed octahedron, with three antimony atoms in the same double layer being slightly closer to each other than the other three. This relatively close proximity allows the density of metallic antimony to reach 6.697g/cm3, but the weak bonding between layers also makes it very soft and fragile.
| Antimony sputtering targets(Sb)产品应用 |
Semiconductor industry