-
-
Sputtering Targets
- Element Sputtering Targets
- Alloy Sputtering Targets
- Oxide Sputtering Targets
- Nitride Sputtering Targets
- Carbide Sputtering Targets
- Fluoride Sputtering Targets
- Silicide Sputtering Targets
- Sulfide Sputtering Targets
- Selenide Sputtering Targets
- Telluride Sputtering Targets
- Antimonide Sputtering Targets
- Phosphide Sputtering Targets
- Special Sputtering Targets Customization
- Sputtering Targets List
-
Evaporation Materials
- Element Evaporation Materials
- Alloy Evaporation Materials
- Oxide Evaporation Materials
- Nitride Evaporation Materials
- Carbide Evaporation Materials
- Fluoride Evaporation Materials
- Phosphide Evaporation Materials
- Telluride Evaporation Materials
- Selenide Evaporation Materials
- Sulfide Evaporation Materials
- Other Evaporation Materials
- Evaporation Materials List
- Compound Semiconductor Materials
- Rare Earth Materials
- Special Alloy
-
Crucible customization
-
Sputtering Targets
- H氢
- He氦
- Li锂
- Be铍
- B硼
- C碳
- N氮
- O氧
- F氟
- Ne氖
- Na钠
- Mg镁
- Al铝
- Si硅
- P磷
- S硫
- Cl氯
- Ar氩
- K钾
- Ca钙
- Sc钪
- Ti钛
- V钒
- Cr铬
- Mn锰
- Fe铁
- Co钴
- Ni镍
- Cu铜
- Zn锌
- Ga镓
- Ge锗
- As砷
- Se硒
- Br溴
- Kr氪
- Rb铷
- Sr锶
- Y钇
- Zr锆
- Nb铌
- Mo钼
- Tc碍
- Ru钌
- Rh铑
- Pd钯
- Ag银
- Cd镉
- In铟
- Sn锡
- Sb锑
- Te碲
- I碘
- Xe氙
- Cs铯
- Ba钡
- LaLu镧系
- Hf铪
- Ta钽
- W钨
- Re铼
- Os锇
- Ir铱
- Pt铂
- Au金
- Hg汞
- Tl铊
- Pb铅
- Bi铋
- Po钋
- At砹
- Rn氡
- La镧
- Ce铈
- Pr镨
- Nd钕
- Pm钷
- Sm钐
- Eu铕
- Gd钆
- Tb铽
- Dy镝
- Ho钬
- Er铒
- Tm铥
- Yb镱
- Lu镥
点击可查看包含对应元素的相关产品信息
科泰新材料可提供“元索周期表”近乎全元素(除放射性元素外)的任意组合材料定制,
部分产品未及时更新,特殊定制需求请咨询客服。
- Al铝
- Ar氩
- As砷
- Ag银
- Au金
- At砹
- Be铍
- B硼
- Br溴
- Ba钡
- Bi铋
- C碳
- Cl氯
- Ca钙
- Cr铬
- Co钴
- Cu铜
- Cd镉
- Cs铯
- Ce铈
- Dy镝
- Eu铕
- Er铒
- F氟
- Fe铁
- Ga镓
- Ge锗
- Gd钆
- H氢
- He氦
- Hf铪
- Hg汞
- Ho钬
- In铟
- I碘
- Ir铱
- K钾
- Kr氪
- Li锂
- LaLu镧系
- La镧
- Lu镥
- Mg镁
- Mn锰
- Mo钼
- N氮
- Ne氖
- Na钠
- Ni镍
- Nb铌
- Nd钕
- O氧
- Os锇
- P磷
- Pd钯
- Pt铂
- Pb铅
- Po钋
- Pr镨
- Pm钷
- Rb铷
- Re铼
- Rn氡
- Ru钌
- Rh铑
- Si硅
- S硫
- Sc钪
- Se硒
- Sr锶
- Sn锡
- Sb锑
- Te碲
- Sm钐
- Ti钛
- Tc碍
- Ta钽
- Tl铊
- Tb铽
- Tm铥
- V钒
- W钨
- Xe氙
- Yb镱
- Y钇
- Zn锌
- Zr锆
当前位置:首页 >> Products >> Sputtering Targets >> Element Sputtering Targets
- 产品类别
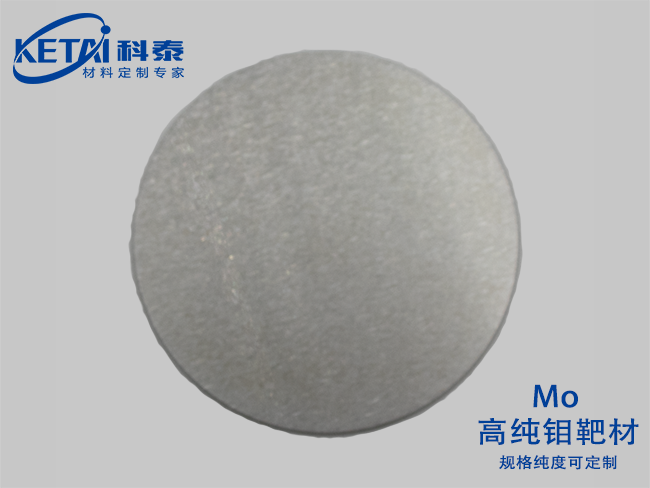
Molybdenum sputtering targets(Mo)
| Molybdenum sputtering targets(Mo)基本信息 | |
| 分子式 | Mo |
| 纯度 | 99.95% |
| CAS号 | 7439-98-7 |
| 摩尔质量 | 95.95 |
| 密度 | 10.2 g/cm³ |
| 熔点 | 2620 ℃ |
| 沸点 | 5560 ℃ |
| 溶解性(水) | |
Molybdenum sputtering targets(Mo)产品概况
Production process of metal molybdenum target:
Powder - Cold Isostatic Pressing - Vacuum Sintering - Rolling - Mechanical Processing
Powder - Vacuum hot pressing sintering - Rolling - Mechanical processing
Molybdenum is a transition metal element located in the fifth cycle and VIB group of the Mendeleev periodic table. Molybdenum has an atomic number of 42 and an atomic weight of 95.95. The electron arrangement in the atom is 1s22s22p63s23p64s23d104p64d55s1. Due to the semi filled state of the valence electron layer orbit, molybdenum is between the lithophile element (8-electron ion configuration) and the cophile element (18-electron ion configuration), exhibiting a typical transition state.
Molybdenum is a silver white metal with an atomic radius of 0.14 nm, an atomic volume of 235.5 px/mol, and a coordination number of 8. The crystal is an Az type body centered cubic crystal system with a spatial group of Oh9. Up to now, no isomerization has been found. The lattice parameters of molybdenum at room temperature range from 0.31467 nm to 0.31475 nm, varying with impurity content. Molybdenum has a high melting point, ranking sixth among the elemental substances in nature, and is known as a refractory metal. The density of molybdenum is 10.23g/cm3, which is about half of that of tungsten (tungsten density 19.36g/cm3). The coefficient of thermal expansion of molybdenum is very low; Molybdenum has a high thermal conductivity. Low molybdenum resistivity: 5.17 at 0 ℃ × 10-10Ω·cm; 24.6 at 800 ℃ × 10-10Ω·cm; 72 at 2400 ℃ × 10-10Ω·cm。 Molybdenum is a paramagnetic material with a specific heat of 242.8J/(kg · K) at 25 ℃. Molybdenum has a relatively high hardness, with a Mohs hardness of 5 to 5.5. The evaporation heat of molybdenum at the boiling point is 594 kJ/mol; The melting heat is 27.6 ± 2.9 kJ/mol; The sublimation heat at 25 ℃ is 659 kJ/mol.
It is extremely difficult for molybdenum to lose seven or eight electrons. This determines that the chemical properties of molybdenum are relatively stable. Molybdenum is stable in air or water at normal or not too high temperatures. Molybdenum is heated in air, and its color begins to change from white to dark gray; When the temperature rises to 520 ℃, molybdenum begins to be slowly oxidized to form Mo2O3; When the temperature rises above 600 ℃, molybdenum is rapidly oxidized to MoO3. Molybdenum is heated to 700~800 ℃ in water vapor and begins to generate MoO2. After further heating, molybdenum dioxide is further oxidized to molybdenum trioxide. Molybdenum can spontaneously ignite in pure oxygen to form molybdenum trioxide.
Molybdenum can react with F2 at room temperature. Molybdenum begins to react with Cl2 at 250 ℃, and molybdenum can react with Cl2 at 700-800 ℃ to form MoCl2. Molybdenum can react with Br2 at a white-hot temperature. The reaction product of molybdenum with halogen can be MoX6 (such as MoF6), MoO2X2 (such as MoO2Cl2), MoOX4 (such as MoOCl4), or MoX. Above 600 ℃, molybdenum begins to embrittle in N2. Molybdenum begins to react with N2 at temperatures above 1500 ℃, and molybdenum reacts with N2 at temperatures above 2400 ℃ to form nitride. However, molybdenum cannot react with H2 until it melts (2622 ℃± 10 ℃). Therefore, in industry, MoO3 is usually reduced with H2 to produce metal molybdenum powder. The reaction process may be as follows: at 450~500 ℃, MoO3 is reduced by H2, and MoO2 is generated after intermediate oxidation states such as Mo5O14, Mo17O47, and Mo4O11 are generated; At 1000-1100 ℃, H2 further reduces MoO2 to metallic molybdenum powder. Molybdenum heated in CO2 can be oxidized to MoO3; The reaction product MoO3 and CO can react and be reduced again to Mo: Mo+3CO2 ←→ MoO3+3CO. Molybdenum carbide can be formed by co heating molybdenum powder or molybdenum oxide in CO or a mixture of CH4 and H2. At 600 ℃, the product is Mo2C, which is brittle, has a density of 8.9g/cm3, and a melting point of 2380 ℃; The product at 800 ℃ is MoC, with a density of 8.4 g/cm3.
| Molybdenum sputtering targets(Mo)产品应用 |
For semiconductor wires and electronic devices