-
-
Sputtering Targets
- Element Sputtering Targets
- Alloy Sputtering Targets
- Oxide Sputtering Targets
- Nitride Sputtering Targets
- Carbide Sputtering Targets
- Fluoride Sputtering Targets
- Silicide Sputtering Targets
- Sulfide Sputtering Targets
- Selenide Sputtering Targets
- Telluride Sputtering Targets
- Antimonide Sputtering Targets
- Phosphide Sputtering Targets
- Special Sputtering Targets Customization
- Sputtering Targets List
-
Evaporation Materials
- Element Evaporation Materials
- Alloy Evaporation Materials
- Oxide Evaporation Materials
- Nitride Evaporation Materials
- Carbide Evaporation Materials
- Fluoride Evaporation Materials
- Phosphide Evaporation Materials
- Telluride Evaporation Materials
- Selenide Evaporation Materials
- Sulfide Evaporation Materials
- Other Evaporation Materials
- Evaporation Materials List
- Compound Semiconductor Materials
- Rare Earth Materials
- Special Alloy
-
Crucible customization
-
Sputtering Targets
- H氢Hydrogen
- He氦Helium
- Li锂Lithum
- Be铍Beryllium
- B硼Boron
- C碳Carbon
- N氮Nitrogen
- O氧Oxygen
- F氟Fluorine
- Ne氖Neon
- Na钠Sodium
- Mg镁Magnesium
- Al铝Aluminium
- Si硅Silicon
- P磷Phosphorus
- S硫Sulfur
- Cl氯Chlorine
- Ar氩Argon
- K钾Potassium
- Ca钙Calcium
- Sc钪Scandium
- Ti钛Titanium
- V钒Vanadium
- Cr铬Chromium
- Mn锰Manganum
- Fe铁Iron
- Co钴Cobalt
- Ni镍Nickel 2
- Cu铜Copper
- Zn锌Zinc
- Ga镓Gallium
- Ge锗Germanium
- As砷Arsenic
- Se硒Selenium
- Br溴Bormine
- Kr氪Krypton
- Rb铷Rubidium
- Sr锶Strontium
- Y钇Yttrium
- Zr锆Zirconium
- Nb铌Niobium
- Mo钼Molybdanium
- Tc碍Technetium
- Ru钌Ruthenium
- Rh铑Rhodium
- Pd钯Palladium
- Ag银Silver
- Cd镉Cadmium
- In铟Inlium
- Sn锡Tin
- Sb锑Antimony
- Te碲Tellurium
- I碘Iodine
- Xe氙Xenon
- Cs铯Caesium
- Ba钡Barium
- LaLu镧系LaLu
- Hf铪Hafnium
- Ta钽Tantalum
- W钨Tungsten
- Re铼Rhenium
- Os锇Osmium
- Ir铱Iridium
- Pt铂Platinum
- Au金Gold
- Hg汞Mercury
- Tl铊Thallium
- Pb铅Lead
- Bi铋Bismuth
- Po钋Polonium
- At砹Astatium
- Rn氡Radon
- La镧Lanthanum
- Ce铈Cerium
- Pr镨Praseodymium
- Nd钕Neodymium
- Pm钷Promethium
- Sm钐Samarium
- Eu铕Europinu
- Gd钆Gadolinium
- Tb铽Terbium
- Dy镝Dysprosium
- Ho钬Holmium
- Er铒Erbium
- Tm铥Thulium
- Yb镱Ytterbium
- Lu镥Lrtetium
Click to view the relevant product information containing the corresponding elements
Ketai new materials can provide customization of any combination of materials with almost all elements (except radioactive elements) in the "yuansuo periodic table". Some products have not been updated in time. Please consult customer service for special customization needs.
- H氢Hydrogen
- He氦Helium
- Li锂Lithum
- Be铍Beryllium
- B硼Boron
- C碳Carbon
- N氮Nitrogen
- O氧Oxygen
- F氟Fluorine
- Ne氖Neon
- Na钠Sodium
- Mg镁Magnesium
- Al铝Aluminium
- Si硅Silicon
- P磷Phosphorus
- S硫Sulfur
- Cl氯Chlorine
- Ar氩Argon
- K钾Potassium
- Ca钙Calcium
- Sc钪Scandium
- Ti钛Titanium
- V钒Vanadium
- Cr铬Chromium
- Mn锰Manganum
- Fe铁Iron
- Co钴Cobalt
- Ni镍Nickel 2
- Cu铜Copper
- Zn锌Zinc
- Ga镓Gallium
- Ge锗Germanium
- As砷Arsenic
- Se硒Selenium
- Br溴Bormine
- Kr氪Krypton
- Rb铷Rubidium
- Sr锶Strontium
- Y钇Yttrium
- Zr锆Zirconium
- Nb铌Niobium
- Mo钼Molybdanium
- Tc碍Technetium
- Ru钌Ruthenium
- Rh铑Rhodium
- Pd钯Palladium
- Ag银Silver
- Cd镉Cadmium
- In铟Inlium
- Sn锡Tin
- Sb锑Antimony
- Te碲Tellurium
- I碘Iodine
- Xe氙Xenon
- Cs铯Caesium
- Ba钡Barium
- LaLu镧系LaLu
- Hf铪Hafnium
- Ta钽Tantalum
- W钨Tungsten
- Re铼Rhenium
- Os锇Osmium
- Ir铱Iridium
- Pt铂Platinum
- Au金Gold
- Hg汞Mercury
- Tl铊Thallium
- Pb铅Lead
- Bi铋Bismuth
- Po钋Polonium
- At砹Astatium
- Rn氡Radon
- La镧Lanthanum
- Ce铈Cerium
- Pr镨Praseodymium
- Nd钕Neodymium
- Pm钷Promethium
- Sm钐Samarium
- Eu铕Europinu
- Gd钆Gadolinium
- Tb铽Terbium
- Dy镝Dysprosium
- Ho钬Holmium
- Er铒Erbium
- Tm铥Thulium
- Yb镱Ytterbium
- Lu镥Lrtetium
current location:Home >> Products >> Sputtering Targets >> Element Sputtering Targets
Select product category:
Element Sputtering Targets Deselect
Deselect
- All products
-
Sputtering Targets
- Element Sputtering Targets
- Alloy Sputtering Targets
- Oxide Sputtering Targets
- Nitride Sputtering Targets
- Carbide Sputtering Targets
- Fluoride Sputtering Targets
- Silicide Sputtering Targets
- Sulfide Sputtering Targets
- Selenide Sputtering Targets
- Telluride Sputtering Targets
- Antimonide Sputtering Targets
- Phosphide Sputtering Targets
- Special Sputtering Targets Customization
- Sputtering Targets List
-
Evaporation Materials
- Element Evaporation Materials
- Alloy Evaporation Materials
- Oxide Evaporation Materials
- Nitride Evaporation Materials
- Carbide Evaporation Materials
- Fluoride Evaporation Materials
- Phosphide Evaporation Materials
- Telluride Evaporation Materials
- Selenide Evaporation Materials
- Sulfide Evaporation Materials
- Other Evaporation Materials
- Evaporation Materials List
- Compound Semiconductor Materials
- Rare Earth Materials
- Special Alloy
-
Crucible customization
- Product category
-
Sputtering Targets
- Element Sputtering Targets
- Alloy Sputtering Targets
- Oxide Sputtering Targets
- Nitride Sputtering Targets
- Carbide Sputtering Targets
- Fluoride Sputtering Targets
- Silicide Sputtering Targets
- Sulfide Sputtering Targets
- Selenide Sputtering Targets
- Telluride Sputtering Targets
- Antimonide Sputtering Targets
- Phosphide Sputtering Targets
- Special Sputtering Targets Customization
- Sputtering Targets List
-
Evaporation Materials
- Element Evaporation Materials
- Alloy Evaporation Materials
- Oxide Evaporation Materials
- Nitride Evaporation Materials
- Carbide Evaporation Materials
- Fluoride Evaporation Materials
- Phosphide Evaporation Materials
- Telluride Evaporation Materials
- Selenide Evaporation Materials
- Sulfide Evaporation Materials
- Other Evaporation Materials
- Evaporation Materials List
- Compound Semiconductor Materials
- Rare Earth Materials
- Special Alloy
-
Crucible customization
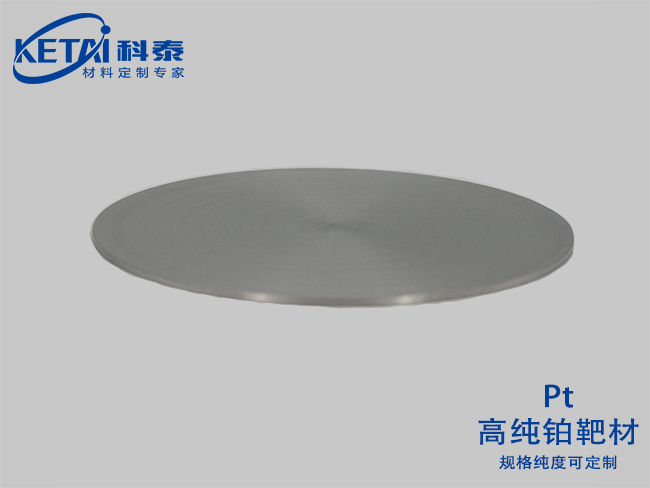
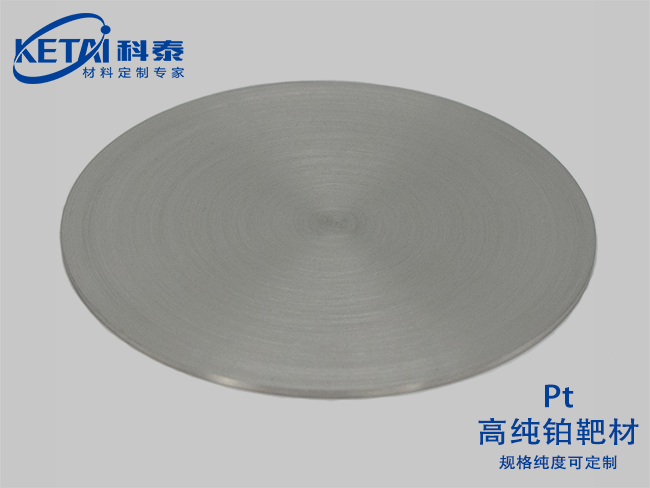
Platinum Sputtering targets(Pt)
| Platinum Sputtering targets(Pt)essential information | |
| Molecular formula | Pt |
| purity | 99.95% |
| CAS No | 7440-06-4 |
| Molar mass | 195.078 |
| density | 21.45g/cm3(20℃) |
| melting point | 1772℃ |
| boiling point | 3827℃ |
| Solubility (water) | |
Platinum Sputtering targets(Pt)Product overview
Platinum is a chemical element, with the chemical symbol Pt. It is one of the precious metals. Its simple substance is commonly known as platinum, and belongs to the platinum series element. Its atomic weight is 195.078, slightly smaller than that of gold. Its atomic number is 78, and it belongs to a transition metal. Melting point 1772 ℃, boiling point 3827 ℃, density 21.45g/cm3 (20 ℃), relatively soft, with good ductility, thermal conductivity, and electrical conductivity. Sponge platinum is a gray spongy substance with a large specific surface area and strong absorption capacity for gases, especially hydrogen, oxygen, and carbon monoxide. Powdered platinum black can absorb large amounts of hydrogen.
Platinum has an inactive chemical property and is stable in air and humid environments. When heated below 450 ℃, a platinum dioxide film forms on the surface, and can react with sulfur, phosphorus, and halogen at high temperatures. Platinum is insoluble in hydrochloric acid, sulfuric acid, nitric acid, and alkali solutions, but it is soluble in aqua regia and molten alkali. The oxidation states of platinum are+2,+3,+4,+5, and+6. It is easy to form coordination compounds, such as [Pt (NH ⏴) ⏴] Cl ⏴, K [Pt (NH ⏴) Cl ⏴].
| Platinum Sputtering targets(Pt)Product application |
Platinum is used as a catalyst in chemical reactions such as hydrogenation, dehydrogenation, isomerization, cyclization, dehydration, dehalogenation, oxidation, and cracking, as well as in the production of sulfuric acid by contact method, nitric acid by ammoxidation, hydrocyanic acid by ammonia and methane, cyclohexane, and vitamins. Reforming naphtha with platinum catalysts can improve the octane number of gasoline products. Platinum and its alloys are resistant to oxidation and corrosion at high temperatures, and are used for making crucibles, evaporating dishes, electrodes, nozzles, reactors, etc. Platinum and platinum rhodium alloys are used as furnace wires and thermocouples for high-temperature furnaces in the metallurgical, glass, and ceramic industries. Platinum is also used to make jewelry.